
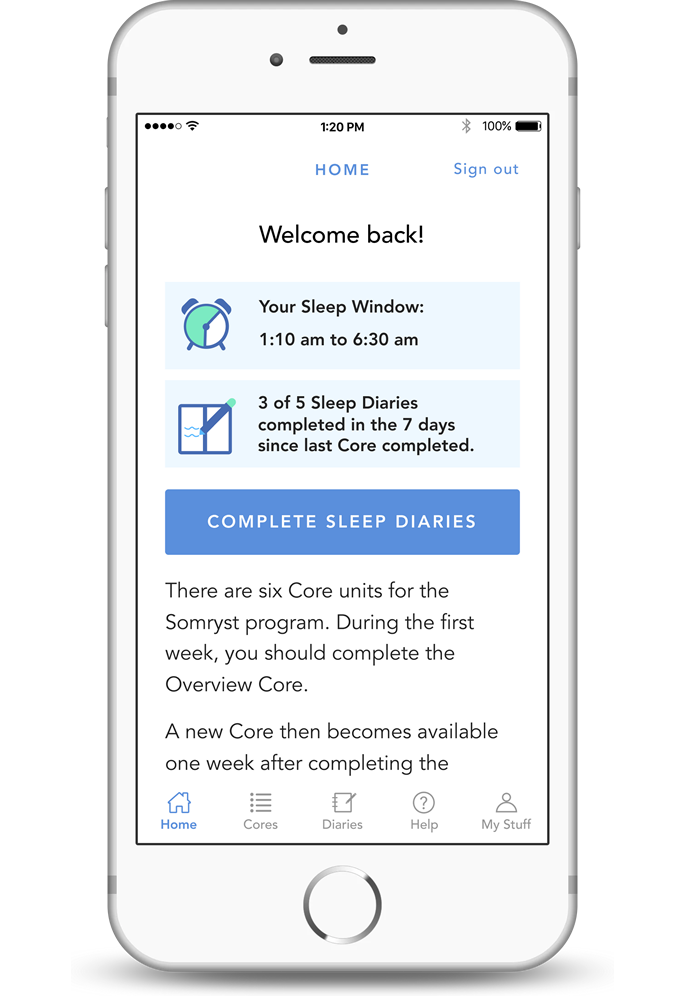
Somryst™ is the first and only prescription digital therapeutic indicated to treat chronic insomnia. Somryst is intended to improve insomnia symptoms by providing neurobehavioral intervention (cognitive behavioral therapy for insomnia – CBT-I) to adults 22 years of age and older with chronic insomnia.
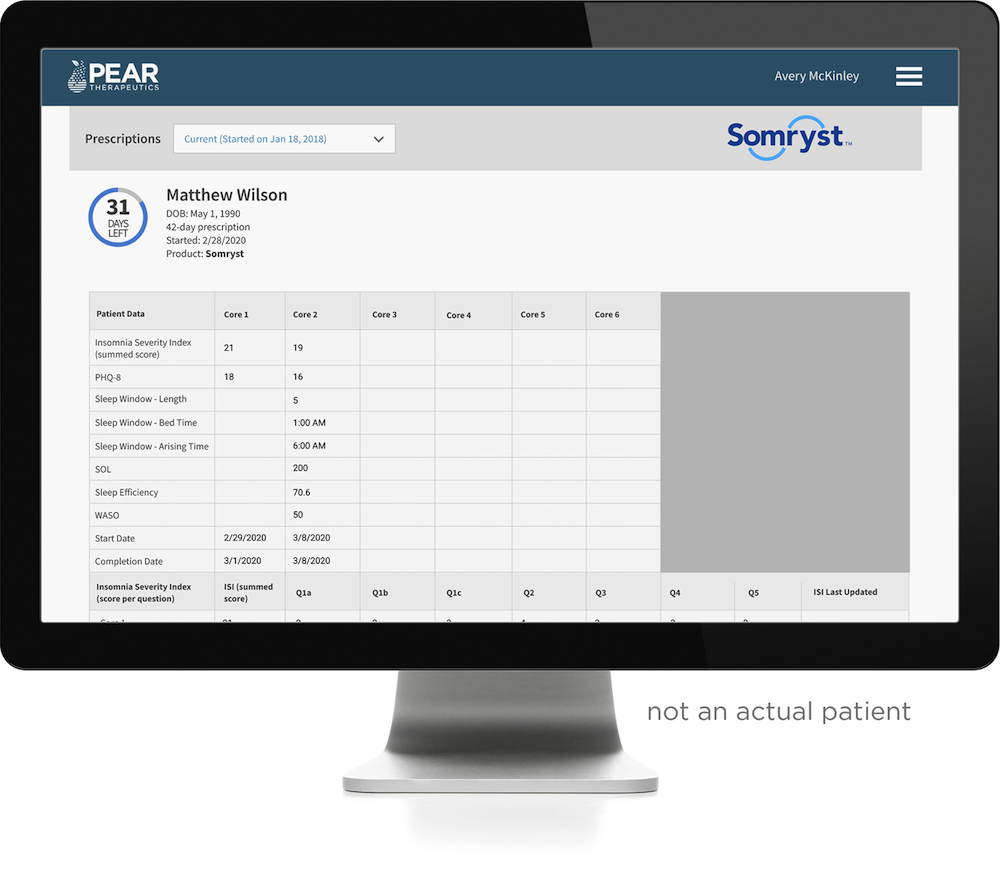
Additionally, Somryst also provides a clinician facing dashboard that allows healthcare providers to track patient treatment and progress. The clinician dashboard displays information about patients’ use of Somryst, including the Insomnia Severity Index (ISI)1, the Patient Health Questionnaire 8 (PHQ-8)2 scores, and sleep metrics derived from nightly sleep diaries.
PATIENT-FACING
APPLICATION
CLINICIAN-FACING
DASHBOARD
Indications for Use
Somryst™ is a prescription-only digital therapeutic intended to provide a neurobehavioral intervention (Cognitive Behavioral Therapy for Insomnia – CBT-I) in patients 22 years of age and older with chronic insomnia. Somryst treats patients with chronic insomnia by improving a patient’s insomnia symptoms.
Who Should Not Use Somryst™ (Contraindications)
Somryst uses sleep restriction and consolidation, limiting the time a patient spends in bed to match the amount of time they sleep. This treatment technique can increase risks to some patients whose pathophysiology may be worsened. Because of this, it is not appropriate for everyone.
Patients with the following conditions or disorders should not use Somryst:
- Any disorder exacerbated by sleep restriction (e.g. bipolar disorder, schizophrenia, other psychotic spectrum disorders)
- Untreated obstructive sleep apnea
- Parasomnias
- Epilepsy
- Individuals at high risk of falls
- Individuals who are pregnant
- Individuals who have any other unstable or degenerative illness judged to be worsened by sleep restriction
delivered as part of Cognitive Behavioral Therapy for Insomnia
Important Safety Information and Indication
Somryst™ is not for everyone. Please use your clinical judgement to determine whether Somryst is right for your patient.
- Somryst is not for emergency use. Please instruct patients to dial 911 or go to the nearest emergency room in the event of a medical emergency.
- Patients should be clearly instructed not to use Somryst to communicate severe, critical, or urgent information to their Health Care Provider.
- Somryst is not meant to be used as treatment without supervision of a Health Care Provider.
- Somryst is not meant to be a substitution for any treatment medication.
- Somryst contains sensitive medical information. Please instruct patients to protect their information by password-protecting their smartphone and tablet, and ensuring no one else may access their device.
- Sleep Restriction (and Consolidation) within Somryst can cause sleepiness, especially in the early stages of using the PDT. Somryst should not be used if the patient needs to be alert or cautious to avoid serious accidents in their job or daily life. Examples include:
- Long-haul truck drivers
- Long-distance bus drivers
- Air traffic controllers
- Operators of heavy machinery
- Some assembly line jobs
- The usage data collected in therapy lessons by Somryst are not intended to be used as a standalone assessment of treatment progress.
Note: In the early stages of treatment, increased daytime sleepiness may be expected, but is usually temporary. Please consult with your Health Care Provider if these experiences do not go away over a few weeks, as it may indicate that you may have another sleep disorder or medical condition other than insomnia. If you have trouble staying awake while performing potentially dangerous tasks (like driving) at any point in the treatment, to avoid these dangerous tasks or stop following the sleep restriction component of the therapy.
Please see the Clinician Brief Summary Instructions for Somryst
ISI1: Charles M. Morin, PhD, Geneviève Belleville, PhD, Lynda Bélanger, PhD, Hans Ivers, PhD, The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response, Sleep, Volume 34, Issue 5, 1 May 2011, Pages 601–608.
PHQ82 Kroenke K., Strein T. W., Spitzer R., Williams J. B., Berry J. T., and Mokdad A. H. (2009). The PHQ-8 as a measure of current depression in the general population. Journal of General Internal Medicine, 114 (1-3), 163-173.
For more information about Somryst click here:
www.somryst.com
For a brief summary of prescribing information on Somryst please see links below:
Clinician Information
